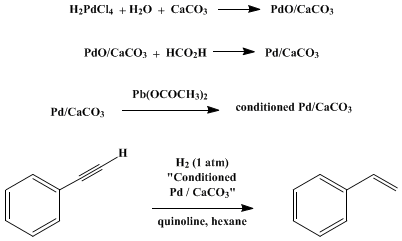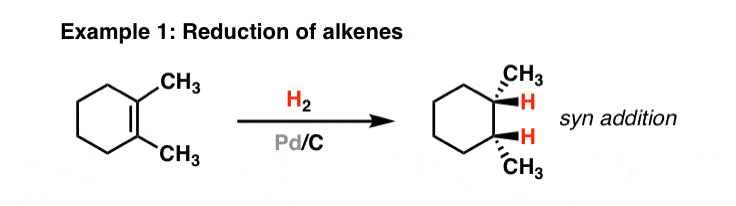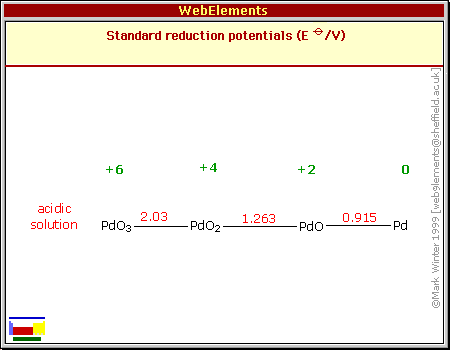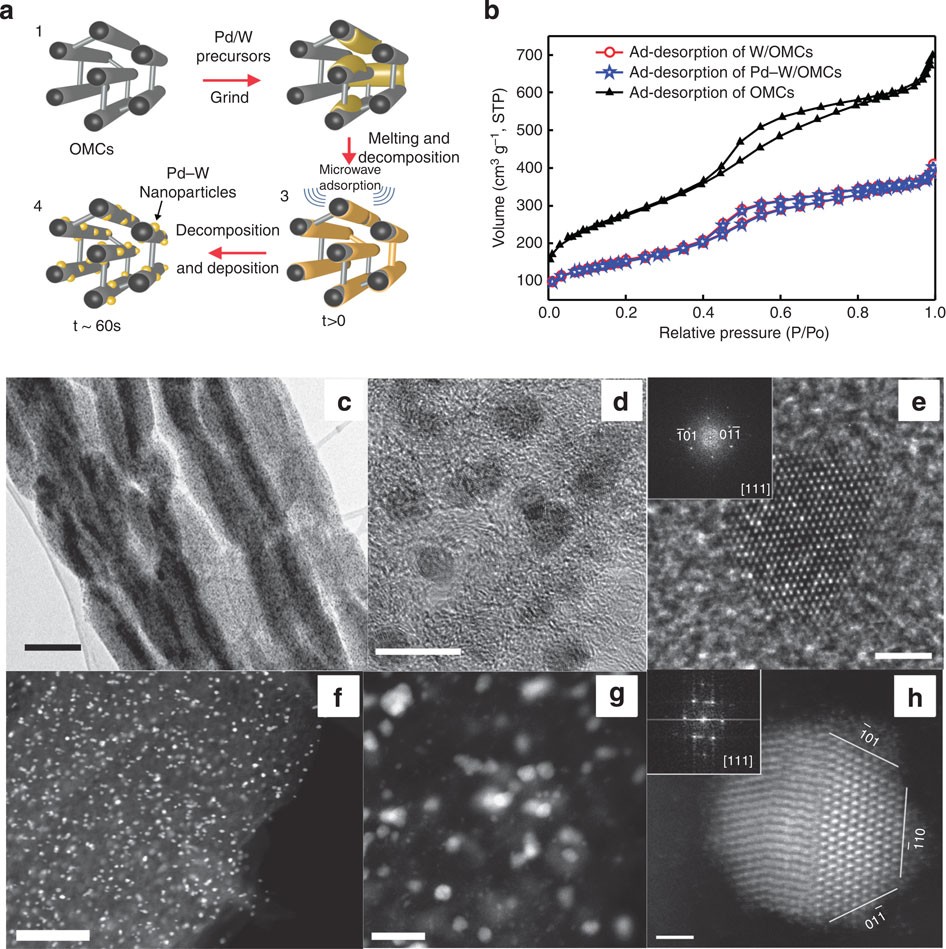
Small palladium islands embedded in palladium–tungsten bimetallic nanoparticles form catalytic hotspots for oxygen reduction | Nature Communications

Molecules | Free Full-Text | Dinuclear Nickel(I) and Palladium(I) Complexes for Highly Active Transformations of Organic Compounds | HTML

The Impact of Palladium(II) Reduction Pathways on the Structure and Activity of Palladium(0) Catalysts - Wei - 2013 - Angewandte Chemie International Edition - Wiley Online Library

Proposed method for palladium reduction to Pd (0) in the periplasm of... | Download Scientific Diagram

The Palladium Acetate‐Catalyzed Microwave‐Assisted Hirao Reaction without an Added Phosphorus Ligand as a “Green” Protocol: A Quantum Chemical Study on the Mechanism - Keglevich - 2017 - Advanced Synthesis & Catalysis -

The ubiquitous cross-coupling catalyst system 'Pd(OAc) 2 '/2PPh 3 forms a unique dinuclear Pd I complex: an important entry point into catalytically c ... - Chemical Science (RSC Publishing) DOI:10.1039/C9SC01847F
![PDF] A hydroquinone based palladium catalyst for room temperature nitro reduction in water | Semantic Scholar PDF] A hydroquinone based palladium catalyst for room temperature nitro reduction in water | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7f50878b12ee80f4266e9a654281d70727457521/2-Table1-1.png)
PDF] A hydroquinone based palladium catalyst for room temperature nitro reduction in water | Semantic Scholar
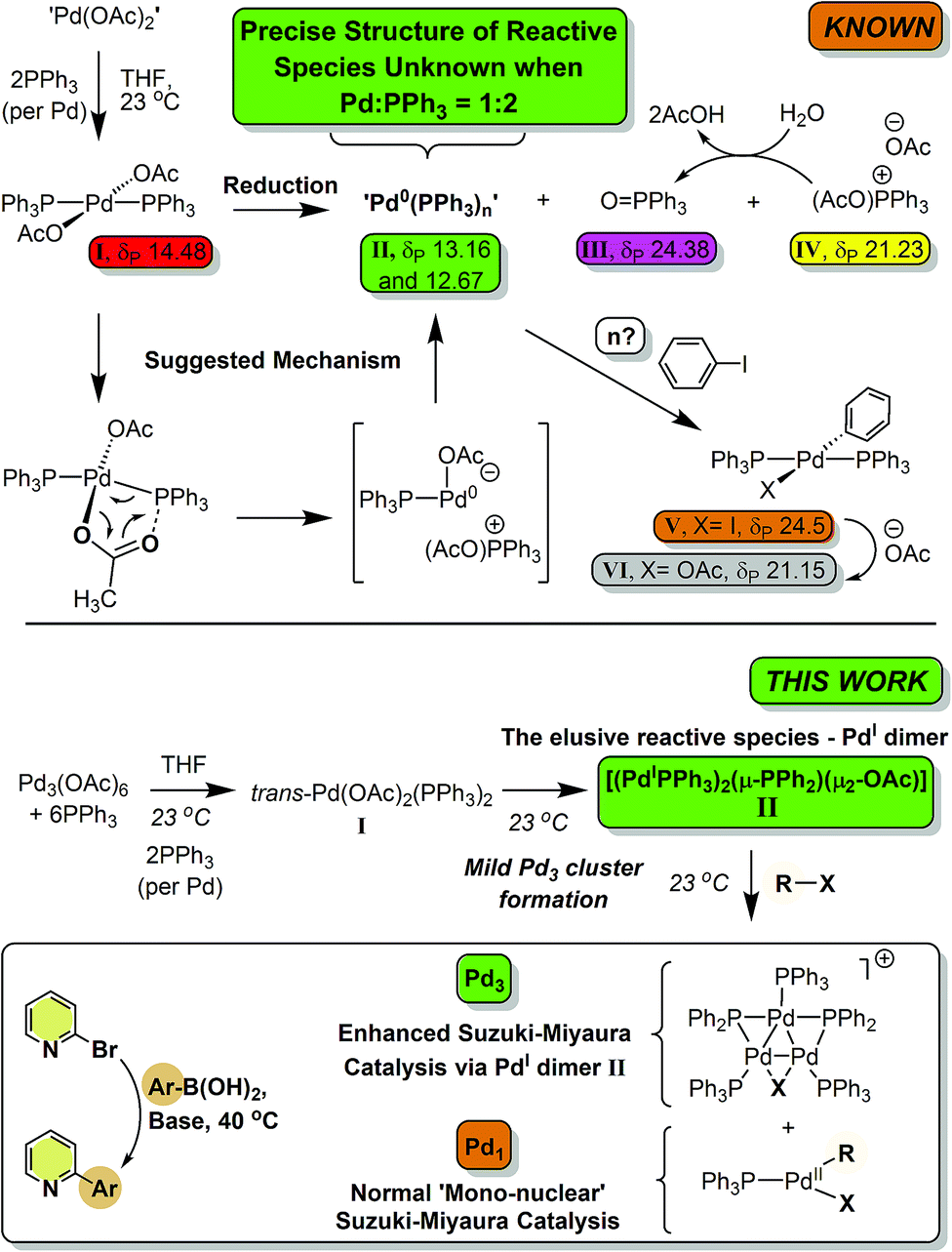
The ubiquitous cross-coupling catalyst system 'Pd(OAc) 2 '/2PPh 3 forms a unique dinuclear Pd I complex: an important entry point into catalytically c ... - Chemical Science (RSC Publishing) DOI:10.1039/C9SC01847F

O 2 insertion into a palladium( ii )-hydride bond: Observation of mechanistic crossover between HX-reductive-elimination and hydrogen-atom-abstraction ... - Chemical Science (RSC Publishing) DOI:10.1039/C0SC00392A

Recovery of Palladium from Spent Activated Carbon-Supported Palladium Catalysts | Johnson Matthey Technology Review

Development of Palladium Precatalysts that Efficiently Generate LPd(0) Active Species - Shaughnessy - 2020 - Israel Journal of Chemistry - Wiley Online Library

Proposed method for palladium reduction to Pd (0) in the periplasm of... | Download Scientific Diagram

The mechanism of palladium(II)-mediated C–H cleavage with mono-N-protected amino acid (MPAA) ligands: origins of rate acceleration


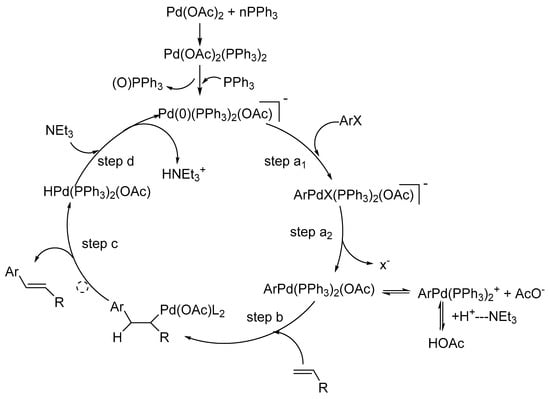

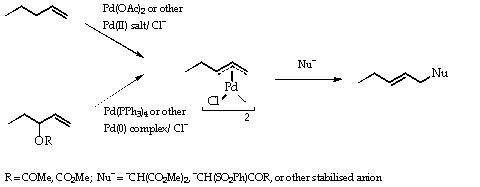
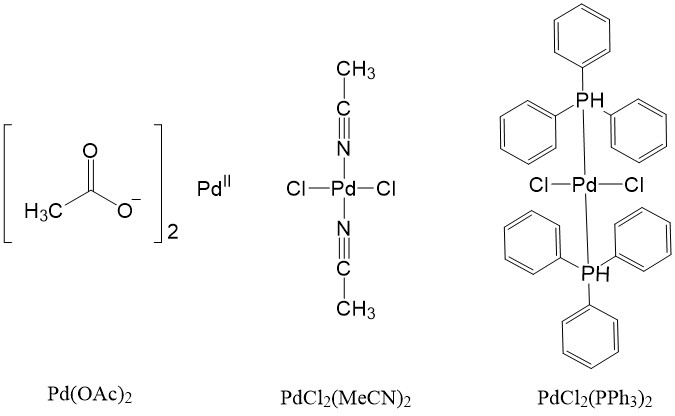

![Structural Characterizations of Palladium Clusters Prepared by Polyol Reduction of [PdCl4]2− Ions Structural Characterizations of Palladium Clusters Prepared by Polyol Reduction of [PdCl4]2− Ions](https://static-01.hindawi.com/articles/jamc/volume-2016/9073594/figures/9073594.fig.001.svgz)