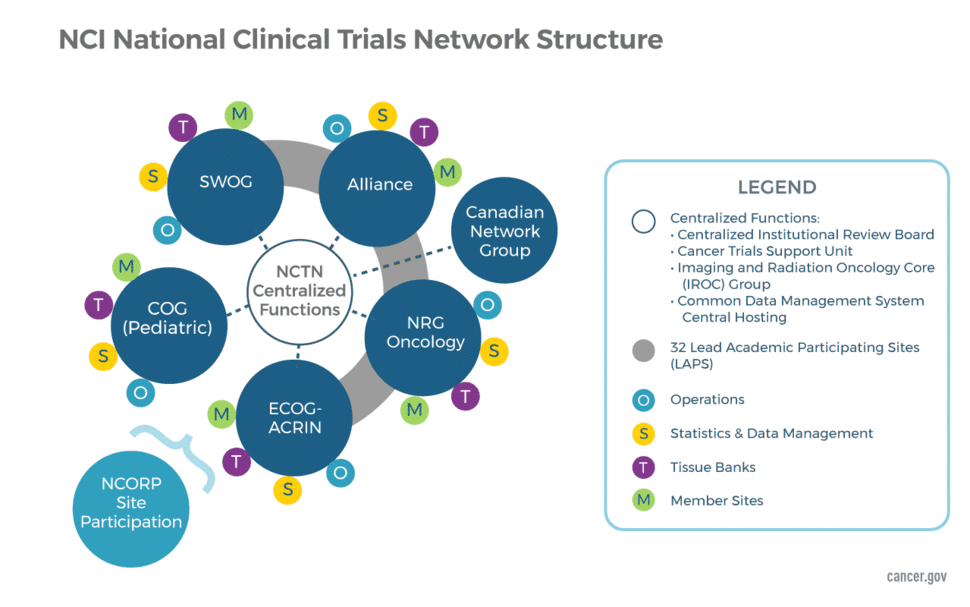
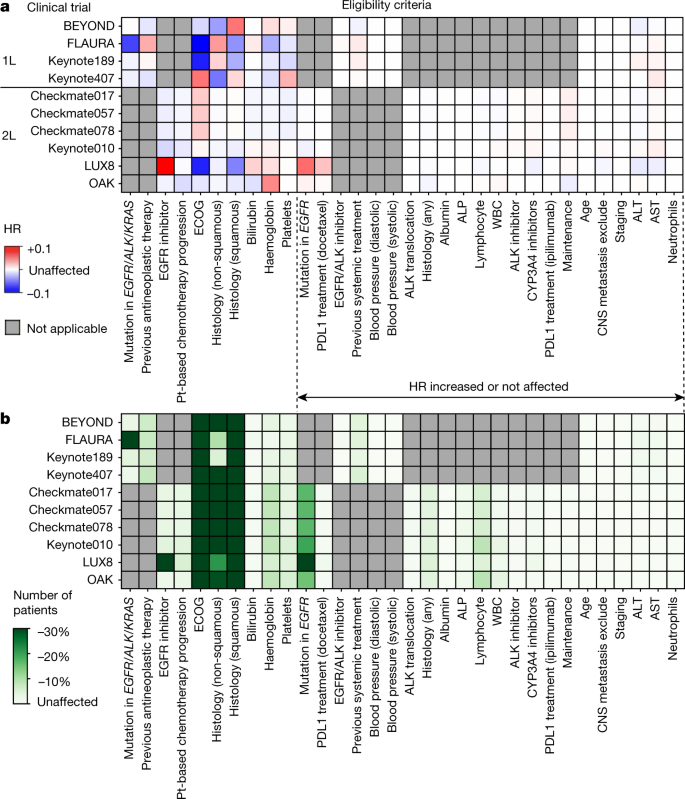
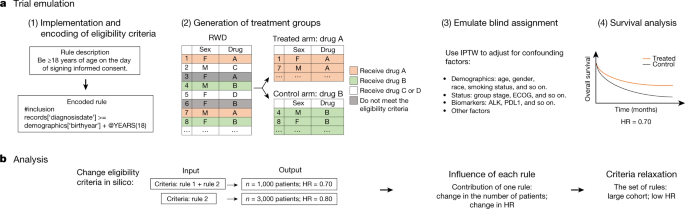
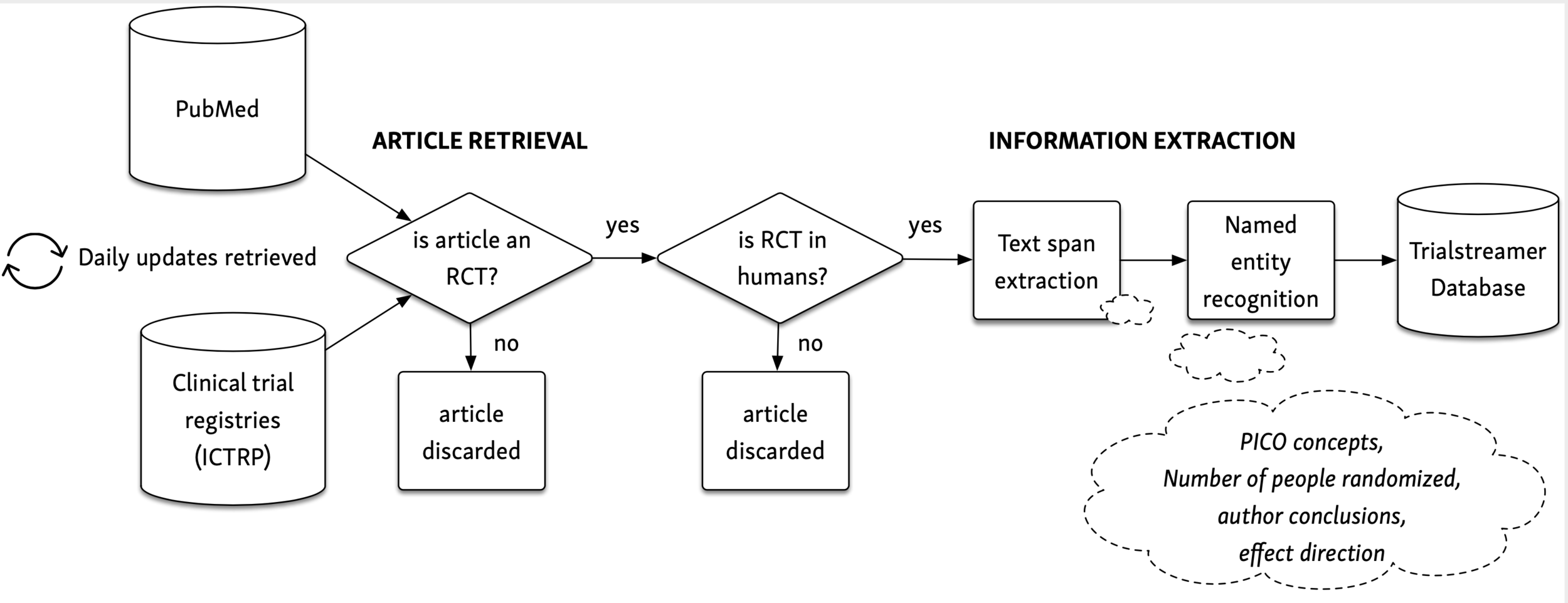
Ongoing Clinical Trials for the Management of the COVID-19 Pandemic: Trends in Pharmacological Sciences

Accessibility of trial reports for drugs stalling in development: a systematic assessment of registered trials – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on

Evidence-informed recommendations to reduce dissemination bias in clinical research: conclusions from the OPEN (Overcome failure to Publish nEgative fiNdings) project based on an international consensus meeting – topic of research paper in

Interpreting Clinical Trial Results Betty Anne Schwarz MSc, BA, RN OHRI Clinical Research Conference Workshop October 16, ppt download
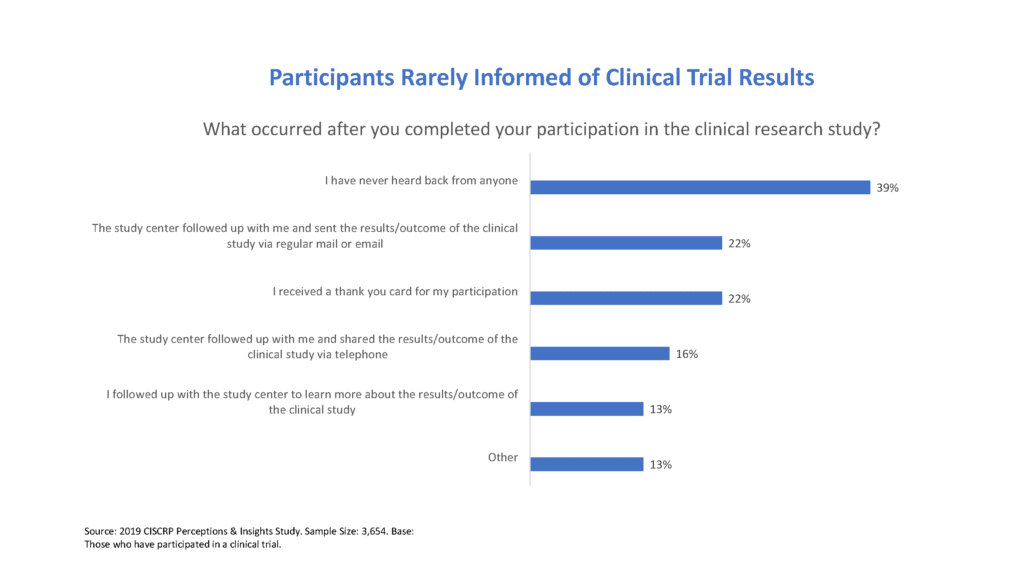
CISCRP's 2019 Perceptions and Insights Study - Center for Information & Study on Clinical Research Participation

Barriers and enablers to locally-led clinical trial conduct in low and middle income countries: strategies for developing locally sustainable health research capacity | Semantic Scholar

Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial - The Lancet Infectious Diseases

PDF) Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016