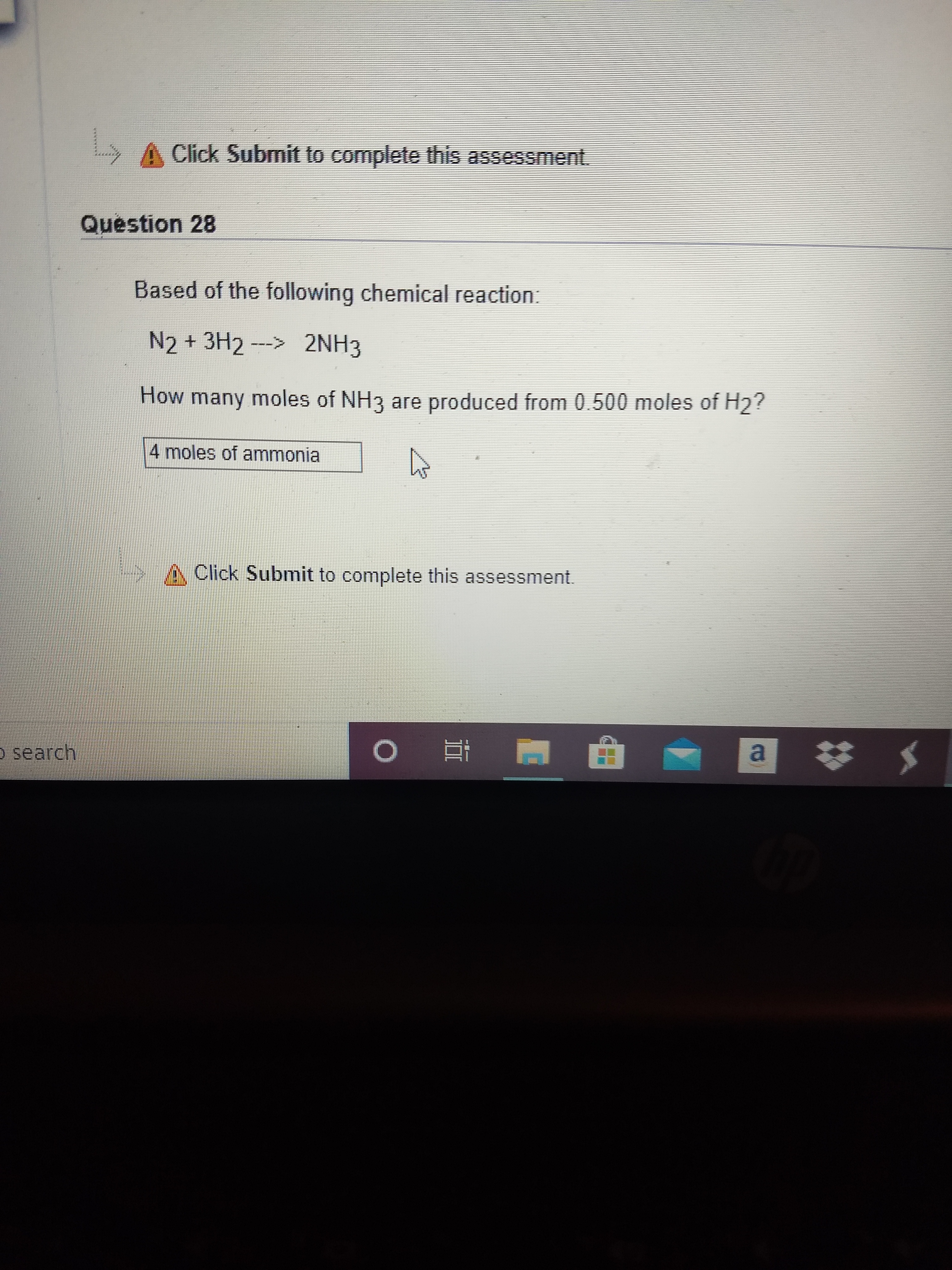
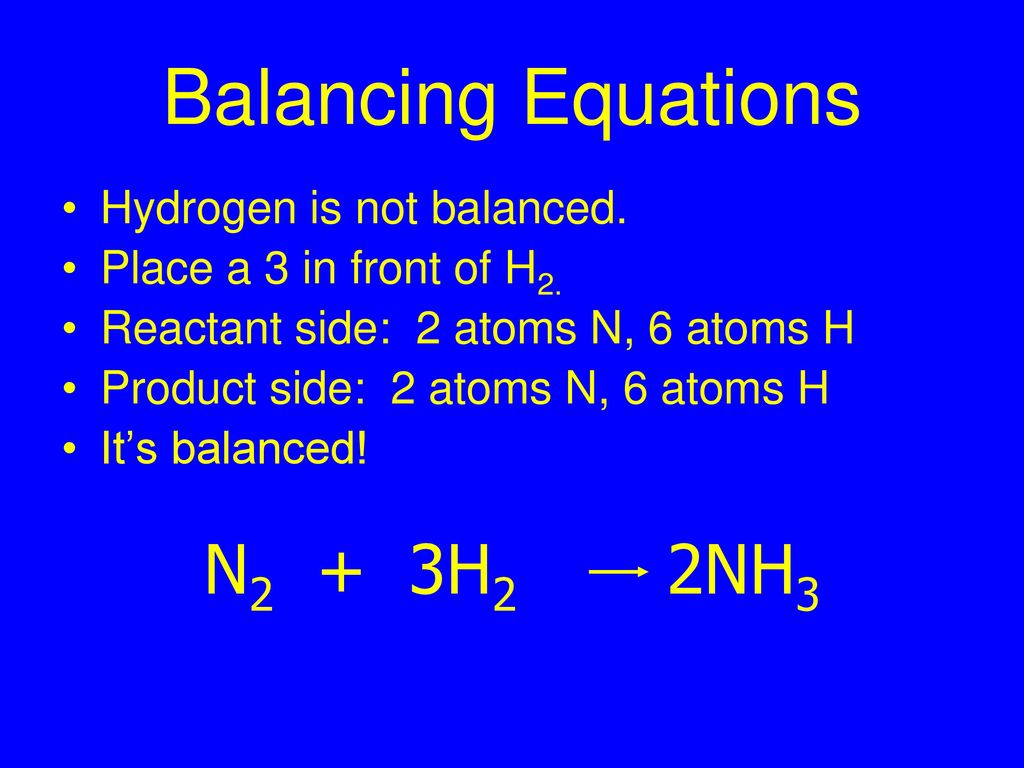
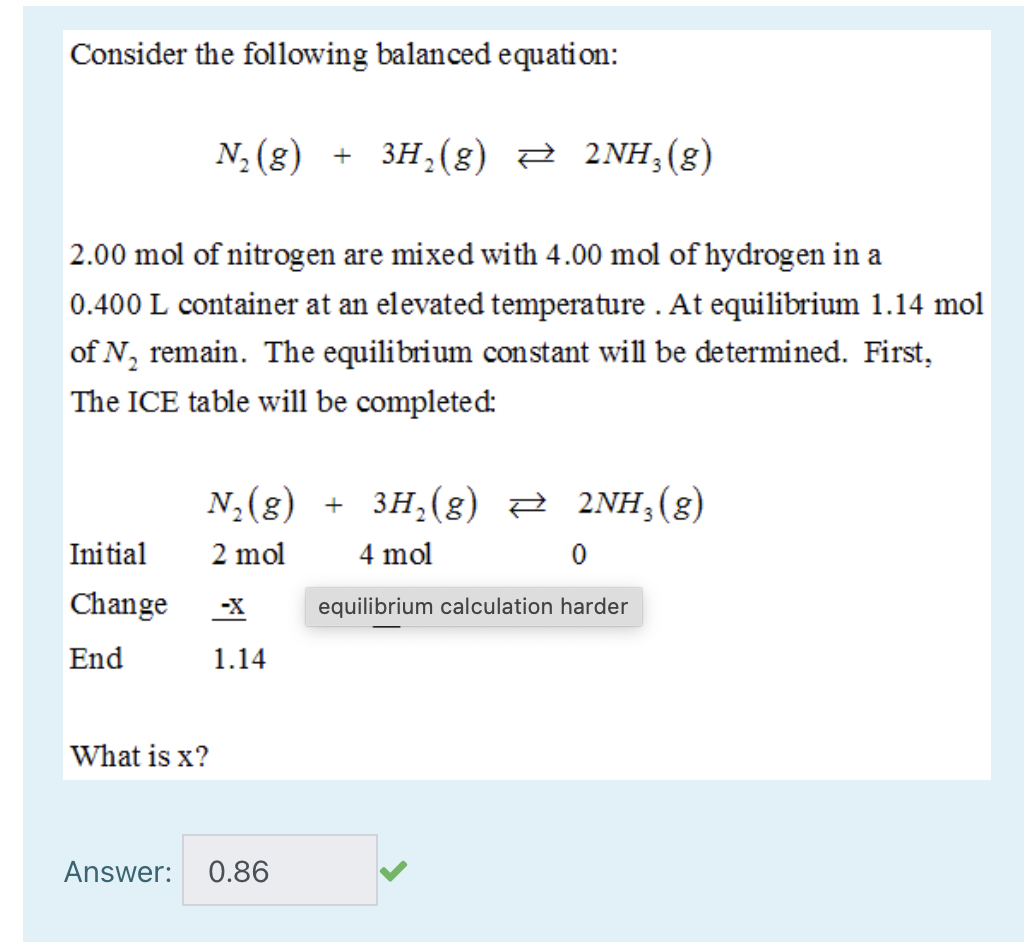
Solved] What is the balanced equation that represents the reaction between hydrogen and nitrogen to make ammonia (NH 3 )? | Course Hero
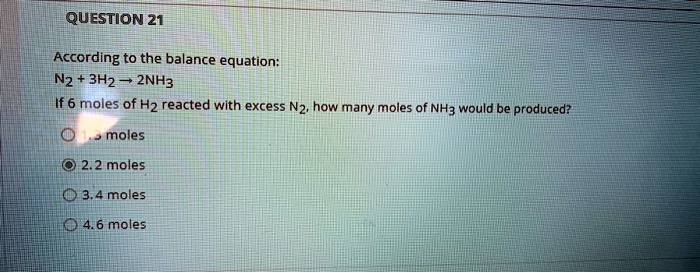
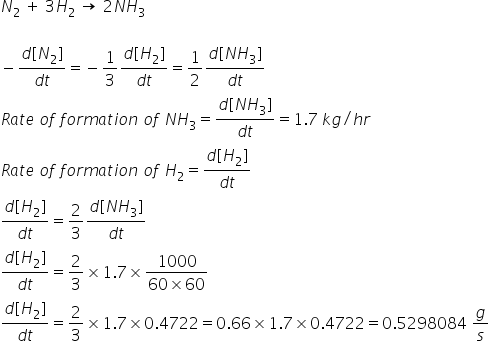
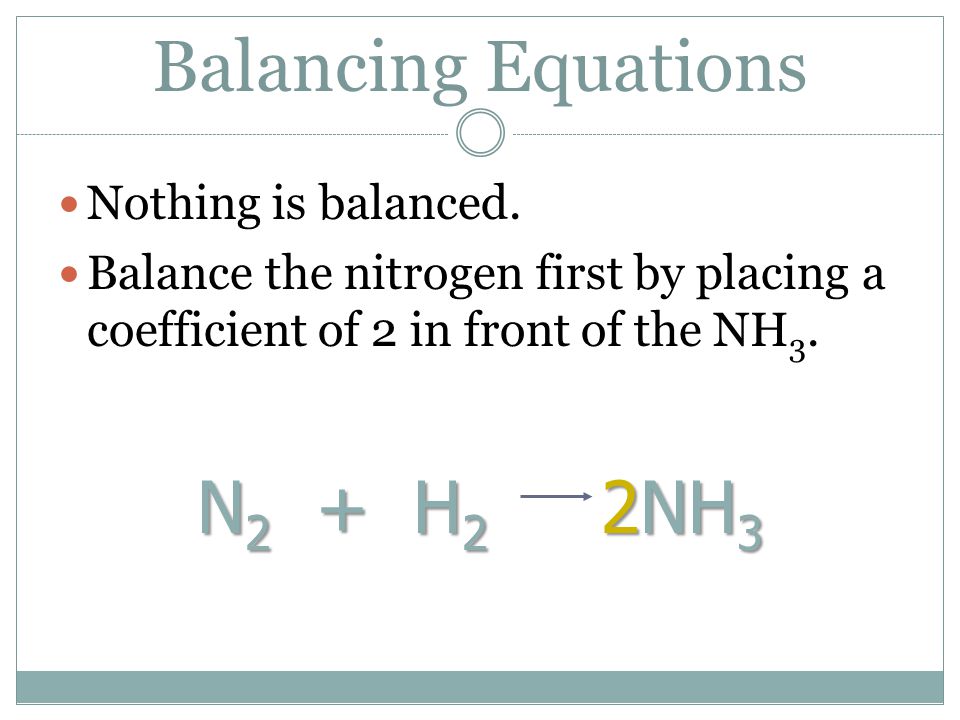
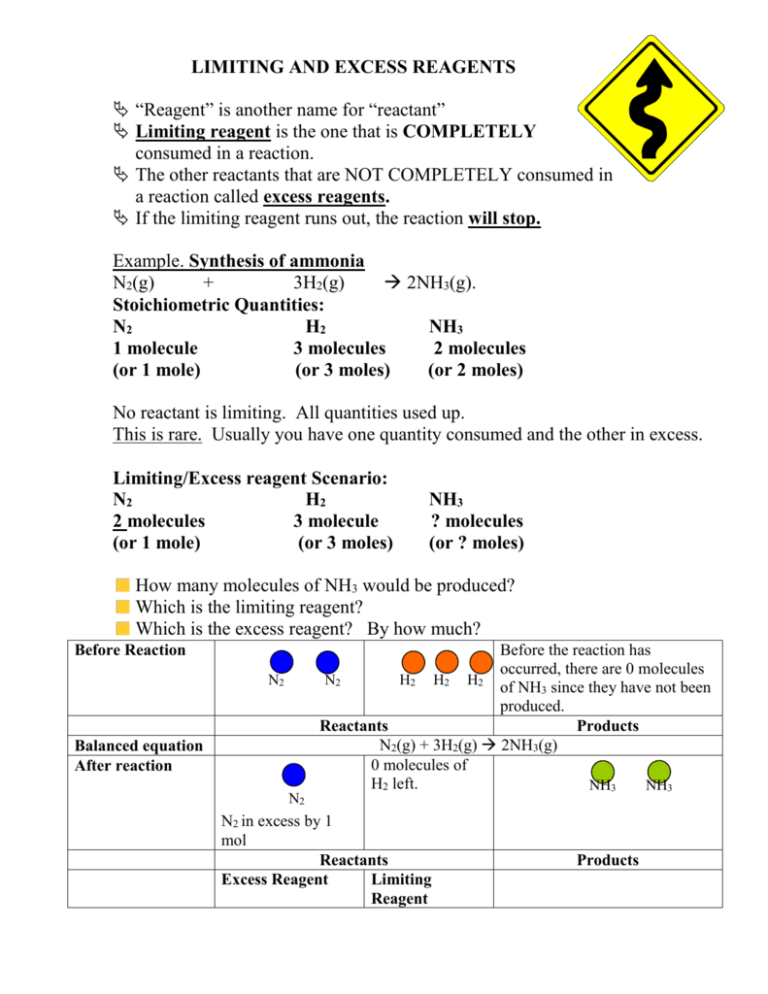
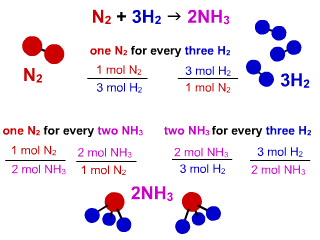
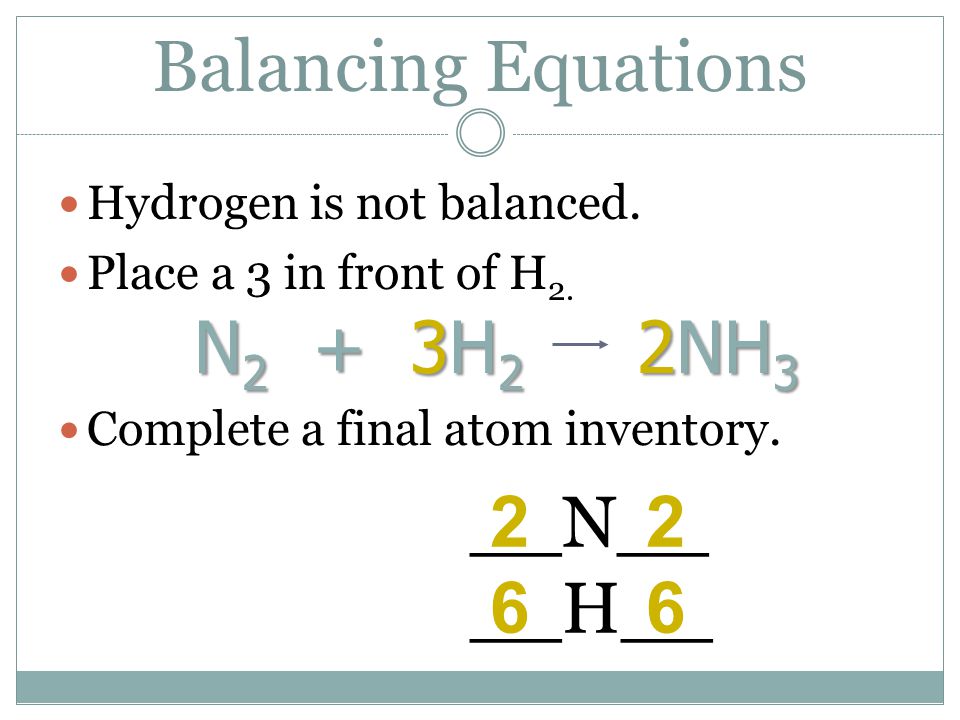
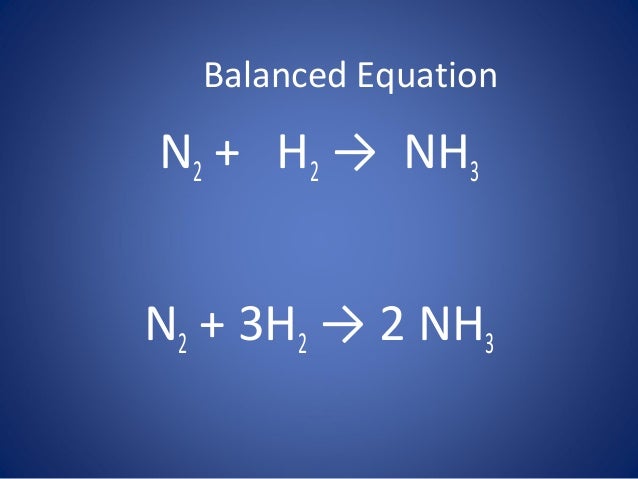
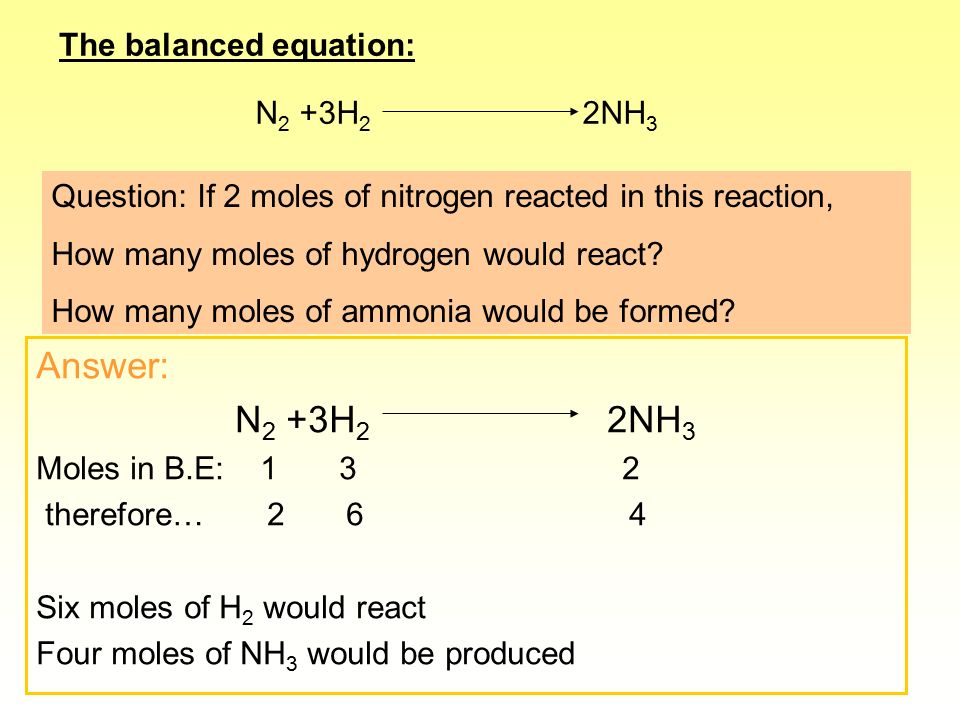
SOLVED:QUESTION 21 According to the balance equation: N2 3H2 2NH3 If 6 moles of Hz reacted with excess Nz, how many moles of _ NHa would be produced? moles 2.2 moles 03.4 moles 014.6 moles